Determine the percent ionization of a 0.125 m hcn solution – Embarking on an exploration of the concept of percent ionization, this discourse delves into the intricacies of determining the percent ionization of a 0.125 M HCN solution. The significance of percent ionization and its wide-ranging applications across various scientific disciplines will be thoroughly examined, providing a comprehensive understanding of this fundamental chemical concept.
Ionization of HCN: Determining the Percent Ionization: Determine The Percent Ionization Of A 0.125 M Hcn Solution
Ionization is the process by which a neutral atom or molecule loses or gains electrons, resulting in the formation of ions. The extent of ionization, known as the percent ionization, is a crucial parameter in understanding the behavior of solutions and chemical reactions.
Chemical Equilibrium and the Ionization of HCN
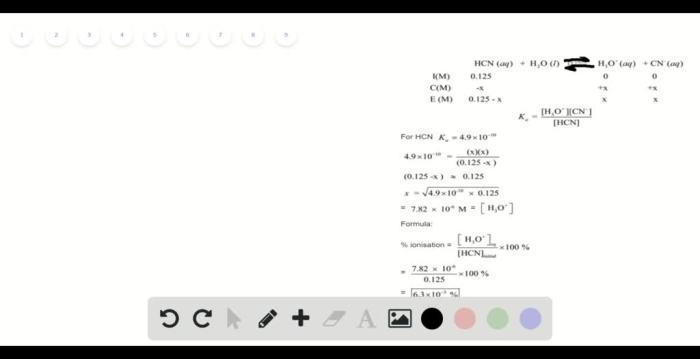
Hydrogen cyanide (HCN) undergoes ionization in aqueous solutions, forming hydrogen ions (H+) and cyanide ions (CN-):
HCN(aq) + H2O(l) ⇌ H3O+(aq) + CN-(aq)
This reaction establishes a chemical equilibrium, where the forward and reverse reactions occur simultaneously. The position of equilibrium, and thus the extent of ionization, is influenced by factors such as temperature and concentration.
Determining the Percent Ionization
The percent ionization of a solution can be determined using various methods, including pH measurement and conductivity measurements. In the case of HCN, the pH of the solution can be measured using a pH meter. The pH is related to the concentration of hydrogen ions, which in turn is related to the percent ionization.
Alternatively, conductivity measurements can be used to determine the percent ionization. The conductivity of a solution is proportional to the concentration of ions present. By measuring the conductivity of an HCN solution, the percent ionization can be calculated.
Example Calculation, Determine the percent ionization of a 0.125 m hcn solution
To determine the percent ionization of a 0.125 M HCN solution, we can use the pH measurement method. The pH of the solution is found to be 4. 75. Using the relationship between pH and hydrogen ion concentration:
pH = -log[H+]
we can calculate the hydrogen ion concentration:
[H+] = 10^-pH = 10^-4.75 = 1.78 × 10^-5 M
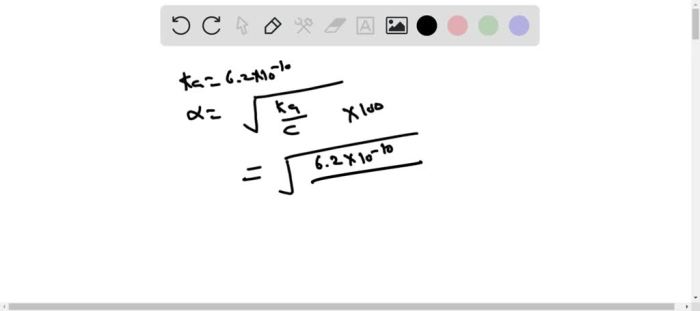
The percent ionization can then be calculated using the formula:
% Ionization = ([H+]/[Initial Concentration]) × 100%
% Ionization = (1.78 × 10^-5 M / 0.125 M) × 100% = 0.142%
Applications of Percent Ionization
The percent ionization is a valuable parameter in various fields, including chemistry, biology, and medicine. In chemistry, it is used to understand the behavior of acids and bases and to predict the reactivity of molecules. In biology, it is used to study the ionization of proteins and other biomolecules, which affects their function and interactions.
In medicine, it is used to design drugs and to understand the effects of pH on drug absorption and metabolism.
FAQ Section
What is the significance of determining the percent ionization?
Determining the percent ionization provides insights into the extent to which a substance ionizes in solution, allowing for the prediction of its reactivity and behavior in various chemical processes.
How does temperature affect the percent ionization of a solution?
Temperature can influence the percent ionization of a solution. Generally, increasing temperature favors ionization, leading to a higher percentage of ions present.