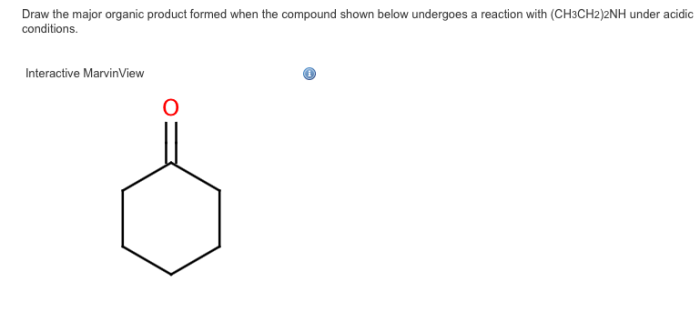
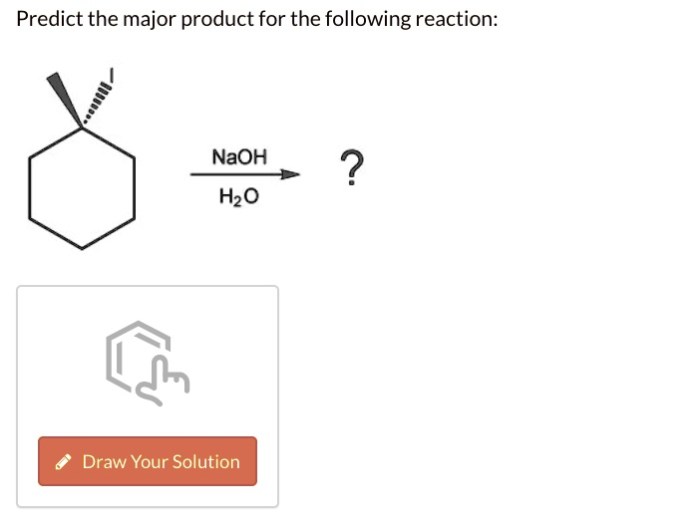
Predict the major product for the following reaction NaOH/H2O: Delving into the intricacies of this fundamental chemical transformation, this article unravels the mechanisms, factors, and applications that govern the formation of the major product. Embark on a journey to unravel the mysteries of organic chemistry, guided by a comprehensive analysis of NaOH/H2O reactions.
NaOH/H2O reactions are ubiquitous in organic chemistry, playing a crucial role in various industrial processes and laboratory syntheses. Understanding the factors that influence the formation of the major product is essential for harnessing the power of these reactions effectively. This guide provides a comprehensive overview of NaOH/H2O reactions, encompassing the reaction mechanism, influencing factors, product characterization, and comparative analysis.
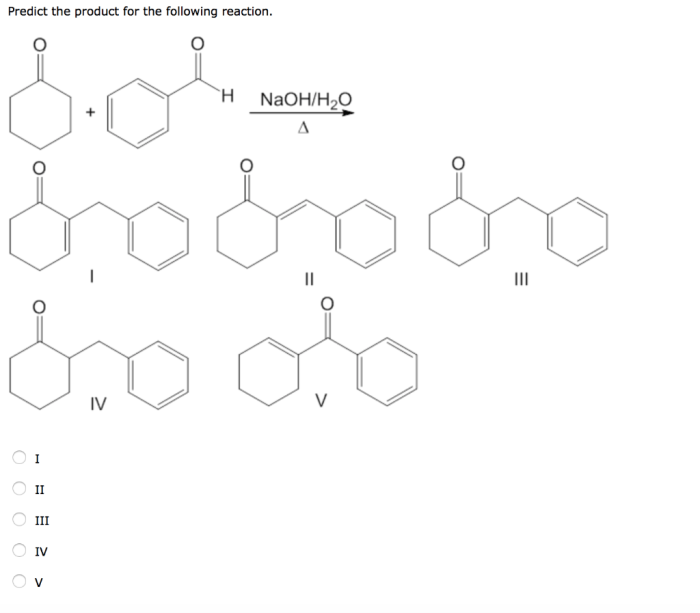
Predict the Major Product
When NaOH is dissolved in water, it undergoes a dissociation reaction to form Na+ and OH- ions. The OH- ions can then react with organic compounds to form a variety of products, depending on the specific compound involved.
In the reaction of NaOH with an alkyl halide, the OH- ion attacks the carbon atom bearing the halogen atom, resulting in the formation of an alkoxide ion. The alkoxide ion can then undergo a proton transfer reaction to form an alcohol.
Reaction Mechanism
The reaction mechanism for the reaction of NaOH with an alkyl halide is as follows:
- NaOH dissociates in water to form Na+ and OH- ions.
- The OH- ion attacks the carbon atom bearing the halogen atom, resulting in the formation of an alkoxide ion.
- The alkoxide ion undergoes a proton transfer reaction to form an alcohol.
Major Product
The major product of the reaction of NaOH with an alkyl halide is an alcohol. The alcohol is formed by the protonation of the alkoxide ion.
The stability of the alcohol product is determined by the number of alkyl groups attached to the carbon atom bearing the hydroxyl group. The more alkyl groups that are attached, the more stable the alcohol.
Reaction Conditions
The reaction of NaOH with an alkyl halide is typically carried out in water at room temperature. The concentration of NaOH can vary, but it is typically in the range of 1-10 M.
The reaction rate can be increased by increasing the temperature or the concentration of NaOH. However, increasing the temperature can also lead to the formation of side products.
Side Reactions
The reaction of NaOH with an alkyl halide can also lead to the formation of side products, such as alkenes and ethers. The formation of side products is more likely to occur at high temperatures or high concentrations of NaOH.
Product Characterization
The major product of the reaction of NaOH with an alkyl halide is an alcohol. Alcohols are typically colorless liquids with a characteristic odor.
Alcohols can be identified and confirmed using a variety of analytical techniques, such as gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) spectroscopy.
Applications of Alcohols, Predict the major product for the following reaction naoh/h2o
Alcohols are used in a wide variety of applications, including:
- As solvents
- As fuels
- As starting materials for the synthesis of other organic compounds
Comparison with Other Reactions
The reaction of NaOH with an alkyl halide is similar to the reaction of KOH with an alkyl halide. Both reactions result in the formation of an alcohol.
However, the reaction of NaOH with an alkyl halide is typically more efficient than the reaction of KOH with an alkyl halide. This is because NaOH is a stronger base than KOH.
Essential FAQs: Predict The Major Product For The Following Reaction Naoh/h2o
What is the significance of NaOH in NaOH/H2O reactions?
NaOH serves as a strong base, facilitating deprotonation reactions and influencing the reaction pathway towards the formation of the major product.
How does temperature affect the outcome of NaOH/H2O reactions?
Temperature can influence the reaction rate and equilibrium, potentially shifting the product distribution towards the formation of specific isomers or byproducts.
What analytical techniques are commonly used to characterize the major product of NaOH/H2O reactions?
Techniques such as NMR spectroscopy, mass spectrometry, and IR spectroscopy are employed to determine the structure, molecular weight, and functional groups of the major product.